Keely
Keely says "When the ether flows from a tube the negative center represents molecular subdivision and carries interstitially between the molecules the lowest order of liberated ozone. This is the first order of ozone, wonderfully refreshing and vitalizing to breathe. The second or atomic order ozone, releases a much higher grade of ozone, too pure for inhalation, for it produces insensibility. The third or etheric order of ozone is used in the carbon register to produce the high vibratory circuit which breaks up cohesion which I recognize as molecular magnetism." [TRANSMUTATION BY SYMPATHETIC VIBRATION]
With his Generator, which was invented for the purpose of multiplication of vibrations, he secured higher frequencies by disturbance of equilibrium of mediums of different specific gravities, air as one, water as the other.
In the disintegration of water in his "Liberator" he produced the "etheric order of ozone." This he is said to have used in a "carbon register" to produce a high vibratory circuit that proved sufficient to break up cohesion, which he states is simply molecular magnetism. At that time he used, in molecular dissociation, one tuning fork of 620 per second, setting chords on the first octave, in atomic separation, two forks, one of 620 and one of 630, setting chords on the second octave, and in etheric separation used three forks, one of 620, one of 630 and one of 12,000, setting chords on the third octave. [VIBRATORY MULTIPLICATION]
Schauberger
[10] The following excerpt from "Pregnant Water" (Schwangeres Wasser) in Implosion Magazine, No. 117, pp. 60-61, explains this process:
"It is a known fact that no free oxygen is present at normal temperatures, but that in the form of ozone it is loosely bound to nitrogen in the ratio of 3O2 to 6N6.
Were it otherwise, then it would not be beneficial to living things. It is only at +40°C (+104°F) that the individual O2 molecules appear, which trigger life-threatening chemical reactions in the human body and are the cause of heat-stroke for example. At about 1,000°C (1,832°F) single-atom molecules of O, identical to the oxygen atom, appear, which naturally have very specific effects. This is why, despite the hermetic seal, the high pressure in high-pressure boilers drops to medium pressure once the above atomic transformation has taken place. Similarly, it is a fact that N (= nitrogen) is not a uniform basic element, but in reality is CH2, i.e. a carbone composed of He3 (helium), wherein two atoms of hydrogen play the role of carrier-substance as it were. Furthermore, it is known that gaseous water and liquid water are quite different things. Gaseous water is OH2 and liquid water (OH2)6. The strong action of gaseous water, for example, follows from this, because two free action quantities or points become active, whereas liquid water has no action quantities, because all the action points are filled with H." [Viktor Schauberger].- Ed. [The Energy Evolution - Harnessing Free Energy from Nature, The Liquefaction of Coal by Means of Cold Flows]
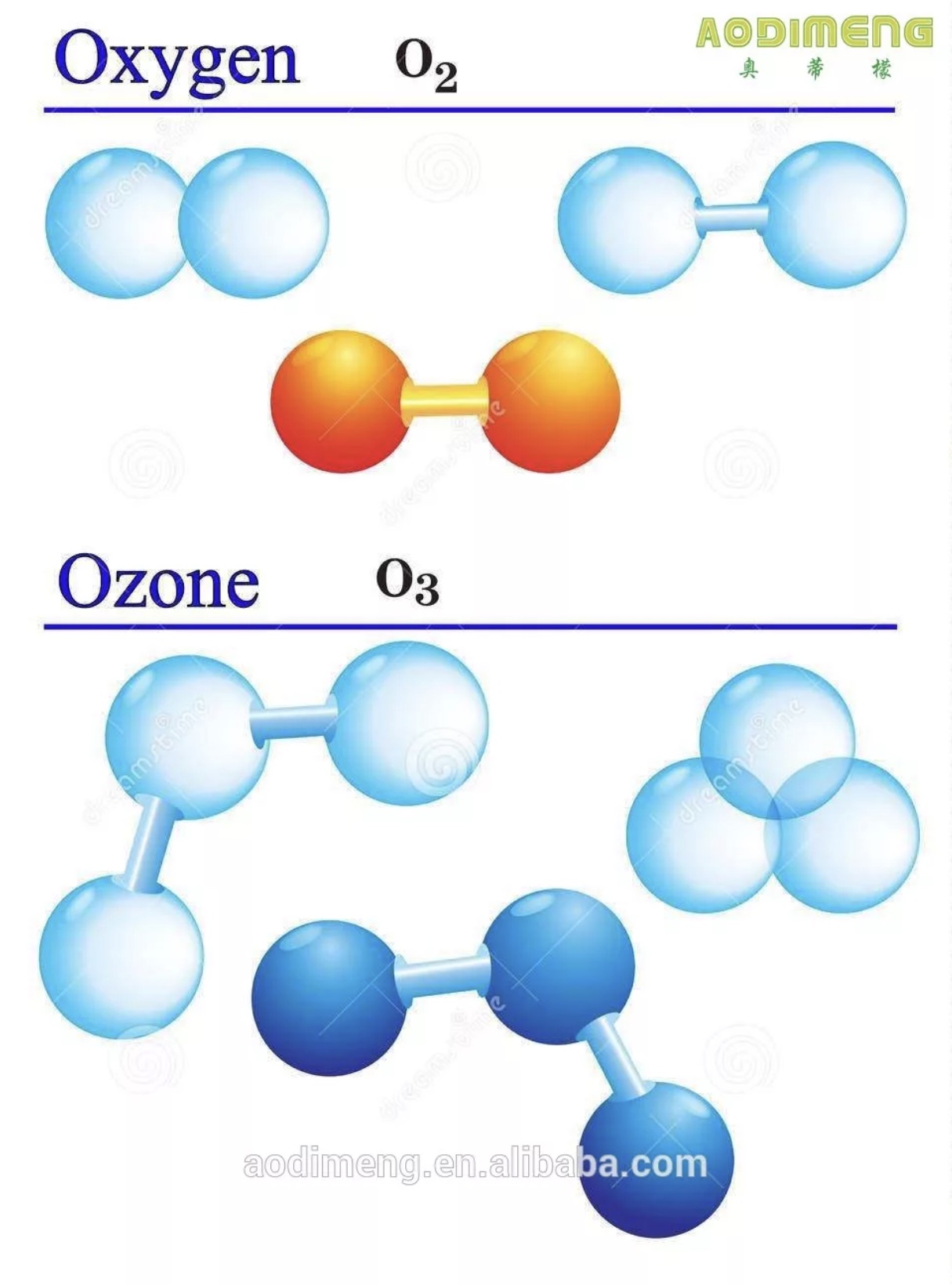
Ozone (O3), or trioxygen, is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope (O2). Ozone in the lower atmosphere is an air pollutant with harmful effects on the respiratory systems of animals and will burn sensitive plants; however, the ozone layer in the upper atmosphere is beneficial, preventing potentially damaging ultraviolet light from reaching the Earth's surface. Ozone is present in low concentrations throughout the Earth's atmosphere. It has many industrial and consumer applications.
History
Ozone, the first allotrope of a chemical element to be recognized, was proposed as a distinct chemical compound by Christian Friedrich Schonbein in 1840, who named it after the Greek verb ozein, from the peculiar odor in lightning storms. The formula for ozone, O3, was not determined until 1865 by Jacques-Louis Soret and confirmed by Schönbein in 1867.
Physical Properties
Ozone is a pale blue gas, slightly soluble in water and much more soluble in inert non-polar solvents such as carbon tetrachloride or fluorocarbons, where it forms a blue solution. At –112°C, it condenses to form a dark blue liquid. It is dangerous to allow this liquid to warm to its boiling point, because both concentrated gaseous ozone and liquid ozone can detonate. At temperatures below 193°C, it forms a violet-black solid.
Most people can detect about 0.01 ppm of Ozone in air where it has a very specific sharp odor somewhat resembling chlorine bleach. Exposure of 0.1 to 1 ppm produces headaches, burning eyes, and irritation to the respiratory passages. Even low concentrations of Ozone in air are very destructive to organic materials such as latex, plastics, and animal lung tissue.
Ozone is diamagnetic, which means that its electrons are all paired. In contrast, O2 is paramagnetic, containing two unpaired electrons.
Structure
According to experimental evidence from microwave spectroscopy, Ozone is a bent molecule, with C2v symmetry (similar to the water molecule). The O – O distances are 127.2 pm. The O - O - O angle is 116.78°. The central atom is sp² hybridized with one lone pair. Ozone is a polar molecule with a dipole moment of 0.5337 D. The bonding can be expressed as a resonance hybrid with a single bond on one side and double bond on the other producing an overall bond order of 1.5 for each side.
Reactions
Ozone is a powerful oxidizing agent, far stronger than O2. It is also unstable at high concentrations, decaying to ordinary diatomic oxygen (with a half-life of about half an hour in atmospheric conditions).
2 O3 → 3 O2
This reaction proceeds more rapidly with increasing temperature and increased pressure. Deflagration of Ozone can be triggered by a spark, and can occur in ozone concentrations of 10 wt% or higher.
Ozone will oxidize most metals (except gold, platinum, and iridium) to oxides of the metals in their highest oxidation state.
Combustion
Ozone can be used for combustion reactions and combusting gases; Ozone provides higher temperatures than combusting in dioxygen (O2). The following is a reaction for the combustion of carbon subnitride which can also cause lower temperatures:
3 C4N2 + 4 O3 → 12 CO + 3 N2
Ozone can react at cryogenic temperatures. At 77 K (ˆ’196 °C), atomic hydrogen reacts with liquid Ozone to form a hydrogen superoxide radical, which dimerizes:
H + O3 → HO2 + O
2 HO2 → H2
Ozone cracking
Ozone gas attacks any polymer possessing olefinic or double bonds within its chain structure, such as natural rubber, nitrile rubber, and styrene-butadiene rubber. Products made using these polymers are especially susceptible to attack, which causes cracks to grow longer and deeper with time, the rate of crack growth depending on the load carried by the product and the concentration of Ozone in the atmosphere. Such materials can be protected by adding antiozonants, such as waxes, which bond to the surface to create a protective film or blend with the material and provide long term protection. Ozone cracking used to be a serious problem in car tires for example, but the problem is now seen only in very old tires. On the other hand, many critical products like gaskets and O-rings may be attacked by Ozone produced within compressed air systems. Fuel lines are often made from reinforced rubber tubing and may also be susceptible to attack, especially within engine compartments where low levels of Ozone are produced from electrical equipment. Storing rubber products in close proximity to DC electric motors can accelerate the rate at which Ozone cracking occurs. The commutator of the motor creates sparks which in turn produce Ozone.
Safety regulations
Due to the strongly oxidizing properties of Ozone, Ozone is a primary irritant, affecting especially the eyes and respiratory systems and can be hazardous at even low concentrations. The Canadian Center for Occupation Safety and Health reports that:
"Even very low concentrations of Ozone can be harmful to the upper respiratory tract and the lungs. The severity of injury depends on both by the concentration of Ozone and the duration of exposure. Severe and permanent lung injury or death could result from even a very short-term exposure to relatively low concentrations."
To protect workers potentially exposed to Ozone, OSHA has established a permissible exposure limit (PEL) of 0.1 ppm (29 CFR 1910.1000 table Z-1), calculated as an 8 hour time weighted average. Higher concentrations are especially hazardous and NIOSH has established an Immediately Dangerous to Life and Health Limit (IDLH) of 5 ppm. Work environments where Ozone is used or where it is likely to be produced should have adequate ventilation and it is prudent to have a monitor for Ozone that will alarm if the concentration exceeds the OSHA PEL. Continuous monitors for Ozone are available from several suppliers.
Elevated Ozone exposure can occur on passenger aircraft, with levels depending on altitude and atmospheric turbulence. U.S. FAA regulations set a limit of 250 ppb with a maximum four-hour average of 100 ppb. Some planes are equipped with Ozone converters in the ventilation system to reduce passenger exposure.
Ultraviolet light
UV Ozone generators, or vacuum-ultraviolet (VUV) Ozone generators, employ a light source that generates a narrow-band ultraviolet light, a subset of that produced by the Sun. The Sun's UV sustains the ozone layer in the stratosphere of Earth.
While standard UV Ozone generators tend to be less expensive, [clarification needed] they usually produce Ozone with a concentration of about 0.5% or lower. Another disadvantage of this method is that it requires the air (oxygen) to be exposed to the UV source for a longer amount of time, and any gas that is not exposed to the UV source will not be treated. This makes UV generators impractical for use in situations that deal with rapidly moving air or water streams (in-duct air sterilization, for example). Production of Ozone is one of the potential dangers of ultraviolet germicidal irradiation. VUV Ozone generators are used in swimming pool and spa applications ranging to millions of gallons of water. VUV Ozone generators, unlike corona discharge generators, do not produce harmful nitrogen by-products and also unlike corona discharge systems, VUV Ozone generators work extremely well in humid air environments. There is also not normally a need for expensive off-gas mechanisms, and no need for air driers or oxygen concentrators which require extra costs and maintenance.
Cold plasma
In the cold plasma method, pure oxygen gas is exposed to a plasma created by dielectric barrier discharge. The diatomic oxygen is split into single atoms, which then recombine in triplets to form ozone.
Cold plasma machines utilize pure oxygen as the input source and produce a maximum concentration of about 5% Ozone. They produce far greater quantities of Ozone in a given space of time compared to ultraviolet production. However, because cold plasma Ozone generators are very expensive, they are found less frequently than the previous two types.
The discharges manifest as filamentary transfer of electrons (micro discharges) in a gap between two electrodes. In order to evenly distribute the micro discharges, a dielectric insulator must be used to separate the metallic electrodes and to prevent arcing.
Some cold plasma units also have the capability of producing short-lived allotropes of oxygen which include O4, O5, O6, O7, etc. These species are even more reactive than ordinary O3.
Special considerations
Ozone cannot be stored and transported like other industrial gases (because it quickly decays into diatomic oxygen) and must therefore be produced on site. Available Ozone generators vary in the arrangement and design of the high-voltage electrodes. At production capacities higher than 20 kg per hour, a gas/water tube heat-exchanger may be utilized as ground electrode and assembled with tubular high-voltage electrodes on the gas-side. The regime of typical gas pressures is around 2 bar absolute in oxygen and 3 bar absolute in air. Several megawatts of electrical power may be installed in large facilities, applied as one phase AC current at 50 to 8000 Hz and peak voltages between 3,000 and 20,000 volts. Applied voltage is usually inversely related to the applied frequency.
The dominating parameter influencing Ozone generation efficiency is the gas temperature, which is controlled by cooling water temperature and/or gas velocity. The cooler the water, the better the Ozone synthesis. The lower the gas velocity, the higher the concentration (but the lower the net ozone produced). At typical industrial conditions, almost 90% of the effective power is dissipated as heat and needs to be removed by a sufficient cooling water flow.
Because of the high reactivity of Ozone, only few materials may be used like stainless steel (quality 316L), titanium, aluminium (as long as no moisture is present), glass, polytetrafluorethylene, or polyvinylidene fluoride. Viton may be used with the restriction of constant mechanical forces and absence of humidity (humidity limitations apply depending on the formulation). Hypalon may be used with the restriction that no water come in contact with it, except for normal atmospheric levels. Embrittlement or shrinkage is the common mode of failure of elastomers with exposure to Ozone. Ozone cracking is the common mode of failure of elastomer seals like O-rings.
Silicone rubbers are usually adequate for use as gaskets in Ozone concentrations below 1 wt%, such as in equipment for accelerated aging of rubber samples.
Incidental production
Ozone may be formed from O2 by electrical discharges and by action of high energy electromagnetic radiation. Certain electrical equipment generate significant levels of Ozone. This is especially true of devices using high voltages, such as ionic air purifiers, laser printers, photocopiers, tasers and arc welders. Electric motors using brushes can generate Ozone from repeated sparking inside the unit. Large motors that use brushes, such as those used by elevators or hydraulic pumps, will generate more Ozone than smaller motors. Ozone is similarly formed in the Catatumbo lightning storms phenomenon on the Catatumbo River in Venezuela, which helps to replenish Ozone in the upper troposphere. It is the world's largest single natural generator of Ozone, lending calls for it to be designated a UNESCO World Heritage Site. (Wikipedia)
An allotropic form of oxygen, the molecule of which consists of three atoms; a common constituent of the atmosphere. It is a powerful oxidizing agent and is used as a disinfectant for swimming pools, etc. [Wellington, Harold; Blakiston's New Gould Medical Dictionary, The Blakiston Company, 1949; 1st edition.]
(For a more complete discussion on this vital substance, see Sands' Primordial Energy. It is the belief of the author that ozone plays a part so important in the overall discussion that its value cannot be over stated.)
Cayce
Q.: "Dr. Johm is quoted as stating that there is a layer of Ozone 50,000 miles from the surface of the earth - is this correct and is it Ozone?"
A.: "That dependent upon what would be termed Ozone. In the sense as that as is thrown off in the essence of the vibratory forces of the earth's sphere and its atmosphere moving through space, leaving as it were - that barrier between space, through which all activity of gravitation acts on through called Ozone, yes. The space varies in the amount - for often there is a trail much farther off, and often much closer." Cayce (195-57)
See Also
Apparatus For Producing Ozone - 568177
Oxygen
Ozone Effects
Ozone Therapy
Tesla Patents 51-100
15.15 - Progressive Dissociation
15.03 - Questions Concerning Dissociation
15.17 - Keely Developing and Controlling Ether
THE PROBLEM OF INCREASING HUMAN ENERGY
15.05 - Relative Diameters in Dissociation
Part 15 - Dissociating Water
15.02 - Liberating Ozone from Water
