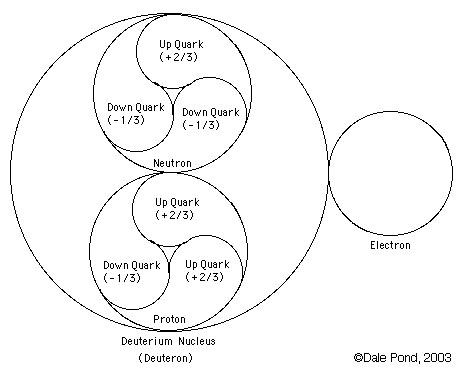
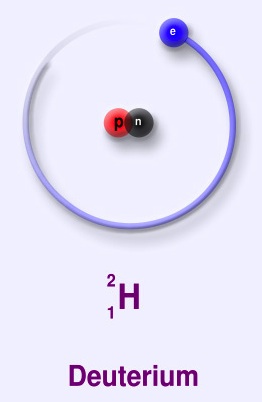
Structure:
Characterisitics:
According to the recommendations of IUPAC Commission on Physical Organic Chemistry [Pure Appl. Chem., 60, 1115-1116 (1988)], the names for hydrogen atoms and ions are the following:
| 1H | 2H | 3H | H | |
| hydron (female) | ||||
| hydride (male) |
Schauberger
[1] See "The Ox and the Chamois" in Nature as Teacher, p.41, Vol. II of the Ecotechnology series. — Ed.
[2] H-substance: here refers to hydrogen or hydrogen-like substances. — Ed.
[3] Phos-elements: It is not quite clear what is intended here, but it may relate in some way to bioluminescence. However, the following three definitions are provided as an aid to interpretation.
PHOSPHOR: A substance which is capable of luminescence, i.e. storing energy (particularly from ionising radiation) and later releasing it in the form of light. If the energy is released after only a short delay (between 10-10 and 10-4 seconds) the substance is called a 'scintillator'.
PHOSPHORUS: P. Element. Atomic weight 30.9738. Atomic number 15. Occurs in several allotropic forms, white phosphorus and red phosphorus being the commonest. The former is a waxy white, very inflammable and poisonous solid. Red phosphorus is a non-poisonous, dark red powder, not very inflammable. The element only occurs in the combined state, mainly as calcium phosphate, CA3(PO4)2, Essential to life; calcium phosphate is the main constituent of animal bones.
PHOSPHATE: Salt of phosphoric acid H3PO4. Phosphates are used as fertilisers to rectify a deficiency of phosphorus in the soil. Note: The editor regrets that he cannot locate the dictionary from which the information was originally sourced.'
[The Energy Evolution - Harnessing Free Energy from Nature, Letter to Werner Zimmermann]
ChatGPT Compares hydrogen to SVP principles and laws [12/29/24]: https://chatgpt.com/share/6771397c-49b8-800d-840e-eae5de509212
ChatGPT further compares hydrogen to SVP principles and laws 12/29/24: [1] https://chatgpt.com/share/6771397c-49b8-800d-840e-eae5de509212
See Also
AI Interpretations of SVP
Hydrogen
Hydrogen - Snell
From Wikipedia, the free encyclopedia.
Deuterium (symbol 2H) is a stable isotope of hydrogen with a natural abundance of one part in 7000 of hydrogen. The nucleus of deuterium (called a deuteron) has one proton and one neutron, whereas a normal hydrogen nucleus just has one proton. Deuterium is also called heavy hydrogen. While it is not an element in its own right, it is often given the symbol D. It occurs naturally as deuterium gas, D2 or 2H2.
deuterium (D), whose nucleus is made up of one proton and one neutron (slightly more massive than a proton, and with no electric charge) bound together as a deuteron (d). About 0.015% of all hydrogen atoms in nature are deuterium.
Deuterium was first detected in 1931 by Harold Clayton Urey, a chemist at Columbia University. Urey earned the 1934 Nobel Prize in Chemistry for this work.
Deuterium is useful in nuclear fusion reactions, as is tritium, because of the larger rate of reaction (or cross section) and high energy yield of the D-T reaction.
Deuterium can replace the normal hydrogen in water molecules to form heavy water (D2O), which was a source of some concern during World War II, as Germany was known to be conducting experiments using heavy water as a nuclear reactor moderator, which might allow them to produce plutonium for an atomic bomb. This led to an important Allied special forces operation to destroy a deuterium production facility in Norway.
Deuterium is frequently used in chemistry and biochemistry as a tracer molecule to study reaction pathways because chemically it behaves identically to ordinary hydrogen, but it can be distinguished from ordinary hydrogen by its mass. Also, because of its greater mass, chemical reactions involving deuterium tend to occur at a slower rate than the corresponding reactions involving ordinary hydrogen.
It has been suggested that deuterium water (heavy water) should be considered toxic because if consumed in isolation it would displace light water and disturb the rate of biochemical reactions in the body. See heavy water for a discussion of this.
The existence of deuterium in stars is one of the arguments in favour of the big bang theory over the steady state theory. Stellar fusion destroys deuterium and there are no known processes other than the big bang itself which produce deuterium.
Canada is the world's leading producer of deuterium as it is needed for the operation of the CANDU reactor.
Data
- density: 0.180 kg.m-3 at STP.
- atomic weight: 2.01363.
"Deuterated ("heavy") water has a higher freezing point than ordinary water, there is indeed a fractionation slightly above zero Celsius, but it does not mean that all heavy water will be frozen.
By holding the temperature at let’s say 1°C, you can get some ice that contains D2O or DHO in a higher concentration than in liquid because the freezing point for H2O is lower.
This way you can reduce the D-concentration of the water with 8-10 ppm in one step. If you wish to achieve further decrease you have to freeze this water in further steps.
According to our knowledge, fractional distillation is the best way to produce DDW in large scale."
This same manufacturer says that home water distillers can at best reduce deuterium concentration by 1 or 2 PPM per pass, which is much less efficient than commercial evaporative methods. But an 8 to 10 drop in PPM from a freezing method (per pass) is considerable (if true), as the early clinical trials in this field suggest that even drops of say 20ppm are potentially clinically significant.
See Also
Etheric Elements
Hydrogen
Particles and Corpuscles
Protium
Table of Quantum Particles
Tritium
water
