Snowflake, crystallized water
Johannes Kepler
“The cause of the six-sided shape of a snowflake is none other than that of the ordered shapes of plants and of numerical constants; and since in them nothing occurs without supreme reason - not, to be sure, such as discursive reasoning discovers, but such as existed from the first in the Creator's design and is presented from the origin to the day in the wonderful nature of animal faculties, I do not believe that even in a snowflake this ordered pattern exists at random.” [Johannes Kepler (1571 - 1630)]
What's cooler than being cool? Ice cold!
The Modeling and Simulations team at Data61 created this animation to show the process of water freezing.
Ever wonder why ice floats or why snowflakes are hexagonal? Well buckle up. Water is a bent little molecule made of a central oxygen atom (in red) and two hydrogen (in white), also known as H2O.
When water is just plain water, the molecules jiggle past each but are quite sticky due to the hydrogen bonds between the oxygen and hydrogen atoms on different water molecules. As the temperature cools, water slows down so much that the hydrogen bonds now become very ordered. Eventually the water molecules no longer slide past each other and the water turns to ice.
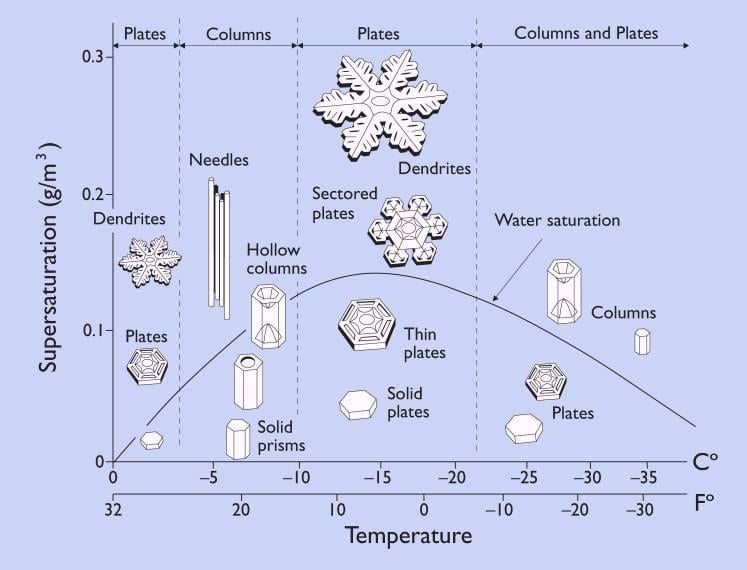
As you can see in the simulation, the ordered ice takes up more volume than for the same amount of water. Being less dense, the ice floats. You can also see that the ordering has hexagonal symmetry. When water vapour turns to ice as in snow formation, this same ordering helps give the snowflake its characteristic hexagonal shape.
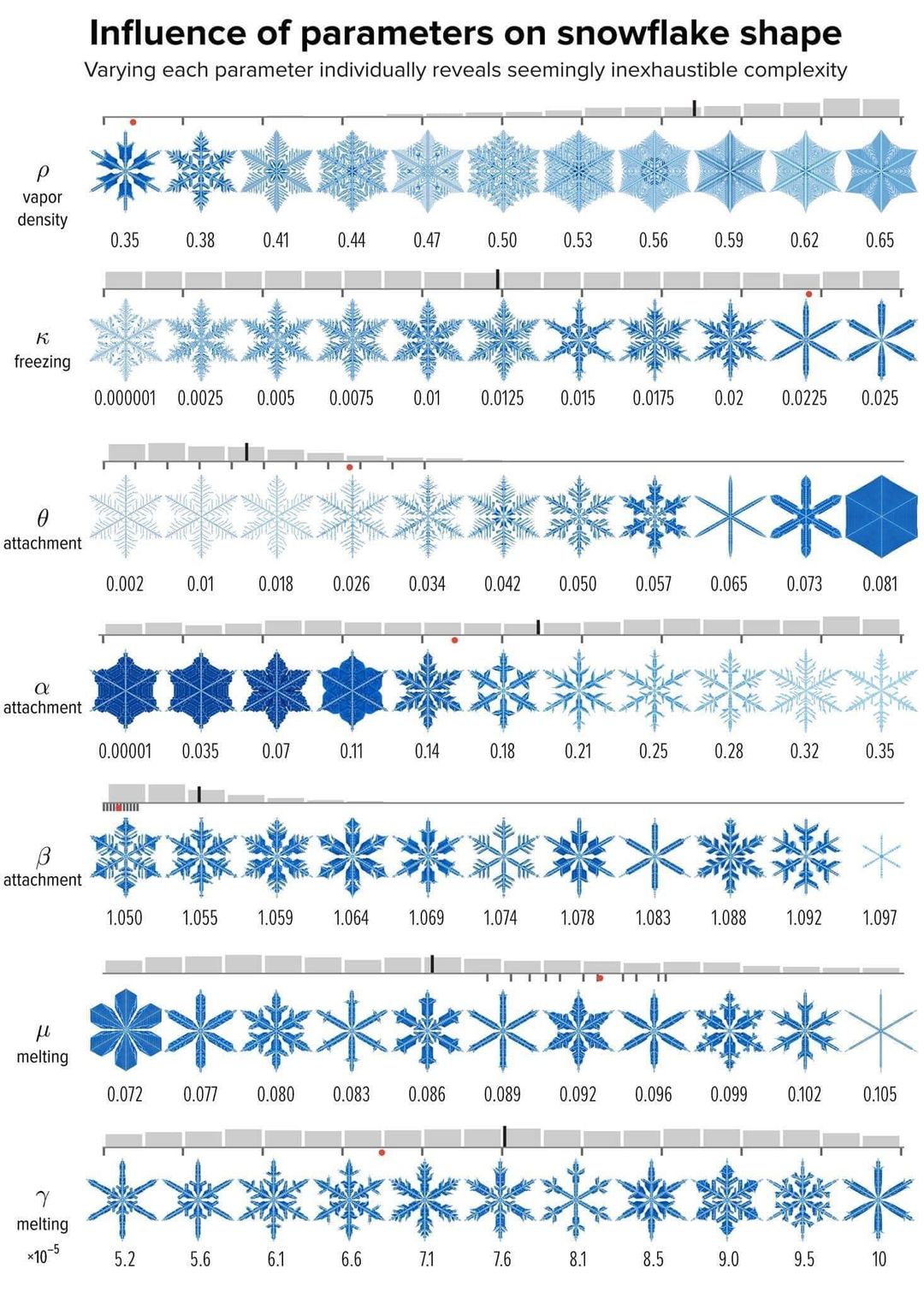
Computing a World of Snowflakes - a must see page on the formation of snowflakes
computing a world of snowflakes
See Also
