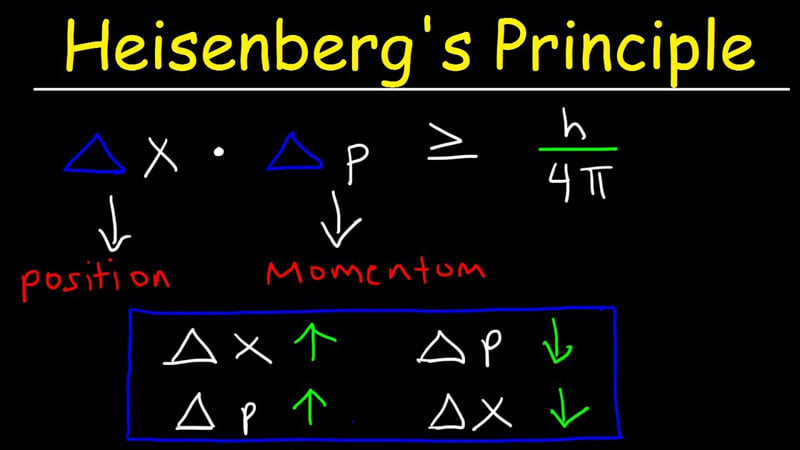
In quantum mechanics, the Heisenberg uncertainty principle states by precise inequalities that certain pairs of physical properties, such as position and momentum, cannot be simultaneously known to arbitrarily high precision. That is, the more precisely one property is measured, the less precisely the other can be measured.
Published by Werner Heisenberg in 1927, the principle means that it is impossible to determine simultaneously both the position and the momentum of an electron or any other particle with any great degree of accuracy or certainty. This is not a statement about researchers' ability to measure the quantities. Rather, it is a statement about the system itself. That is, a system cannot be defined to have simultaneously singular values of these pairs of quantities. The principle states that a minimum exists for the product of the uncertainties in these properties that is equal to or greater than one half of the reduced Planck constant (ħ = h/2π). (wikipedia)
Werner Heisenberg introduced a key idea in quantum mechanics called the uncertainty principle. It says that you cannot know both the exact position and exact momentum of a particle at the same time. This limit is not due to weak tools or poor experiments. It is built into the nature of matter itself.
The principle comes from wave behavior. Particles at tiny scales act like waves. A wave that is tightly focused in space has a wide range of possible momenta. A wave with a clear single momentum spreads out in space. Because matter behaves in this dual way you cannot lock both values at once.
If a scientist tries to measure a particles position with great precision the momentum becomes more uncertain. If the scientist tries to get a very precise momentum the position becomes more spread out. This trade off appears no matter how advanced the measurement device may be.
The uncertainty principle shows that the small world does not follow the same rules as the larger world. Objects like cars or planets have positions and speeds that we can measure clearly. Tiny particles do not work this way. Their values shift with the act of observation.
Heisenbergs idea changed the direction of physics. It helped build the base of quantum theory. It also showed that nature has limits that cannot be broken. The principle guides research in atoms light and particles and remains a central rule in modern science.
See Also
approximation
DIRECT OBSERVATION UNRELIABLE
method of exhaustion
Observation
Thought
Werner Heisenberg
