Also known as Standard electrode potential
In electrochemistry, the standard electrode potential is the measure of the individual potential of a reversible electrode at standard state, i.e., with solutes at an effective concentration of 1 mol dm?3 and gases at a pressure of 1 atm. Values for standard electrode potentials are most often tabulated at 25 °C and with reference to a standard hydrogen electrode (SHE). The standard electrode potential has the symbol E° or E? (with a superscript plimsoll character, pronounced "standard" or "nought" and is an intensive property.
The basis for an electrochemical cell, such as the galvanic cell, is always a redox reaction which can be broken down into two half-reactions: oxidation at anode (loss of electron) and reduction at cathode (gain of electron). Electricity is generated due to electric potential difference between two electrodes. This potential difference is created as a result of the difference between individual potentials of the two metal electrodes with respect to the electrolyte. (Reversible electrode is an electrode that owes its potential to changes of a reversible nature, in contrast to electrodes used in electroplating which are destroyed during their use.)
Although the overall potential of a cell can be measured, there is no simple way to accurately measure the electrode/electrolyte potentials in isolation. The electric potential also varies with temperature, concentration and pressure. Since the oxidation potential of a half-reaction is the negative of the reduction potential in a redox reaction, it is sufficient to calculate either one of the potentials. Therefore, standard electrode potential is commonly written as standard reduction potential. Wikipedia, Standard electrode potential
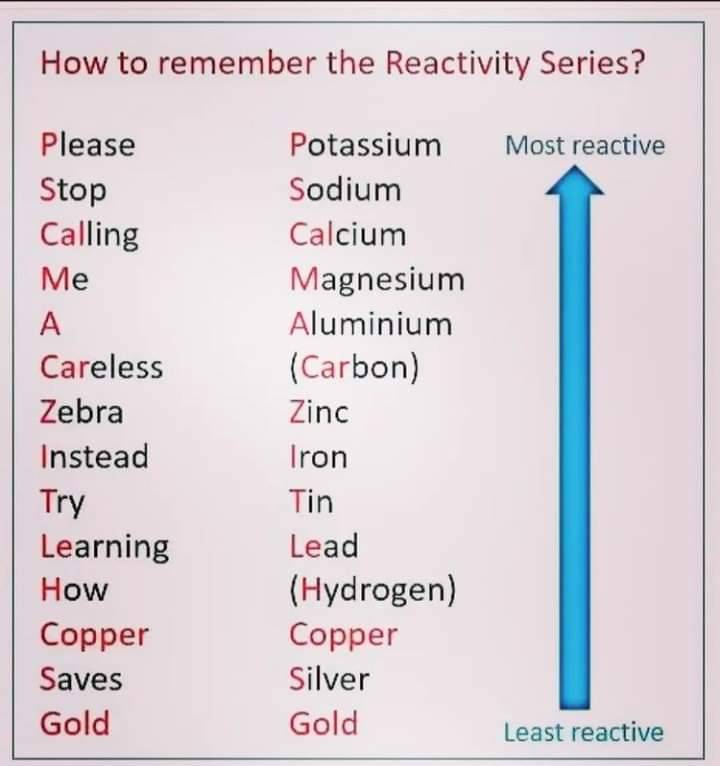
| K | Na | Ca | Mg | Al | Mn | Zn | Fe | Ni | Sn | Pb | <-H-> | Cu | Hg | Ag | Au | Pt | C |
| 701+ | 601+ | 702+ | 602+ | 603+ | 703D+ | 703C- | 703E+ | 703E- | 803A- | 903A- | 401+ | 703D- | 903C- | 803D- | 903D- | 903F- | 504++ |
RULES:
1) Each metal will displace from their salts all the others situated to the right of it in the electromotive series.
2) All metals situated to the left of hydrogen will displace it from acids, while those situated to the right of it will not.
3) The farther two metals are from one another, the higher the voltage of a galvanic cell made up of them. (Electrodes made of metals from the left of H and right of H will provide the greastest potentials.)
NOTES:
Stainless steel, when used as both electrodes simultaneously, will produce one of the LEAST biased potentials and hence produce the least amount of Hydrogen and Oxygen.
Potassium (K) and Sodium (Na) are explosive in water, use with care and only as alloys with other metals.
1) The weight of a substance deposited by electrolysis is proportional to the quantity of electricity passing through the solution and is quite independent of any other factors.
2) During electrolysis equal quantities of electricity liberate equivalent quantities of substances from various chemical compounds.
See Also
Battery
Capacitor
Electrolysis
electromotive force
Faraday
Galvanic Cell
HHO
Volta Alessandro
12.04 - Locked Potentials and the Square Law
